Our group is dedicated to the development of advanced battery materials by gaining profound insights into charge transfer and structural evolution behavior across various space and time scales. A battery chemistry generally consists of several complex ion and electron transfer processes by which an electrochemical reaction can happen. The key parts enabling battery operation are electrode materials and eletrolytes in which ions and electrons can be accommodated or transferred. Thus, our primary focus revolves around comprehending the structure, redox mechanisms, mass diffusion, and charge transfer phenomena occurring in electrode and electrolyte materials, as well as their interfaces. Concurrently, we engage in the design of novel electrode and electrolyte materials suitable for diverse battery applications, including Li(Na)-ion batteries, solid-state batteries, and beyond Li-ion battery technologies. Further details regarding these efforts are provided below.
(1) Cathode materials for Li(Na)-ion batteries
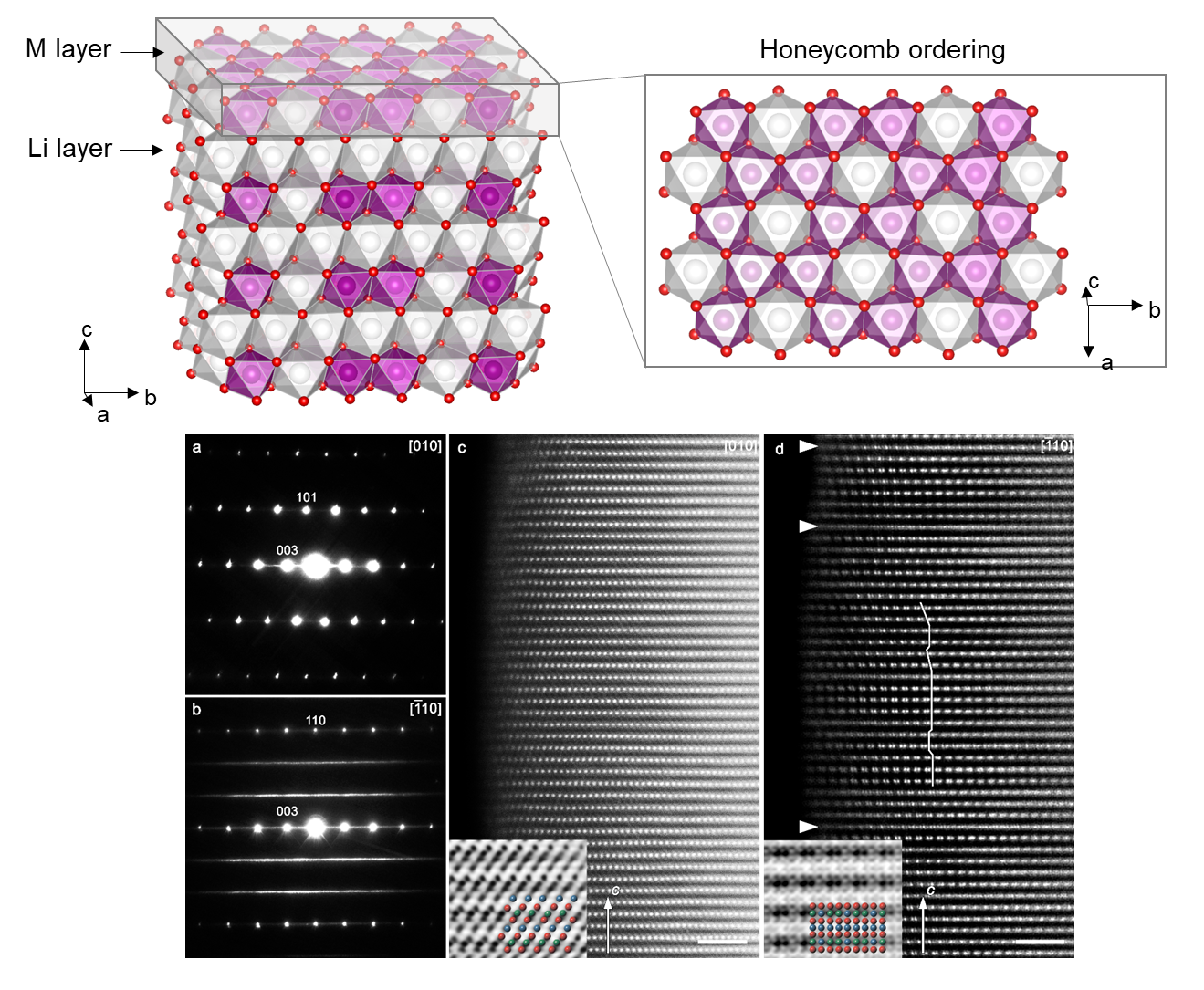
Cathode materials play a pivotal role in the cost of Li(Na)-ion batteries and have been the primary driving force behind the advancement of rechargeable battery technology for several decades. While conventional cathode materials like LiCoO2 and LiFePO4 are widely used, our research primarily focuses on investigating next-generation high-capacity cathode materials. Notably, we concentrate on the exploration of cutting-edge Li-rich oxide materials and Ni-rich layered cathodes, which exhibit exceptional energy storage capabilities. Furthermore, we employ a novel approach by combining these two materials, known as the "Li-rich Ni-rich" concept, to enhance cathode design. Leveraging our expertise in Li-ion technology, we also extend our investigations to Na-ion batteries to explore potential Na cathodes.
(2) All-solid-state batteries: materials and interfaces
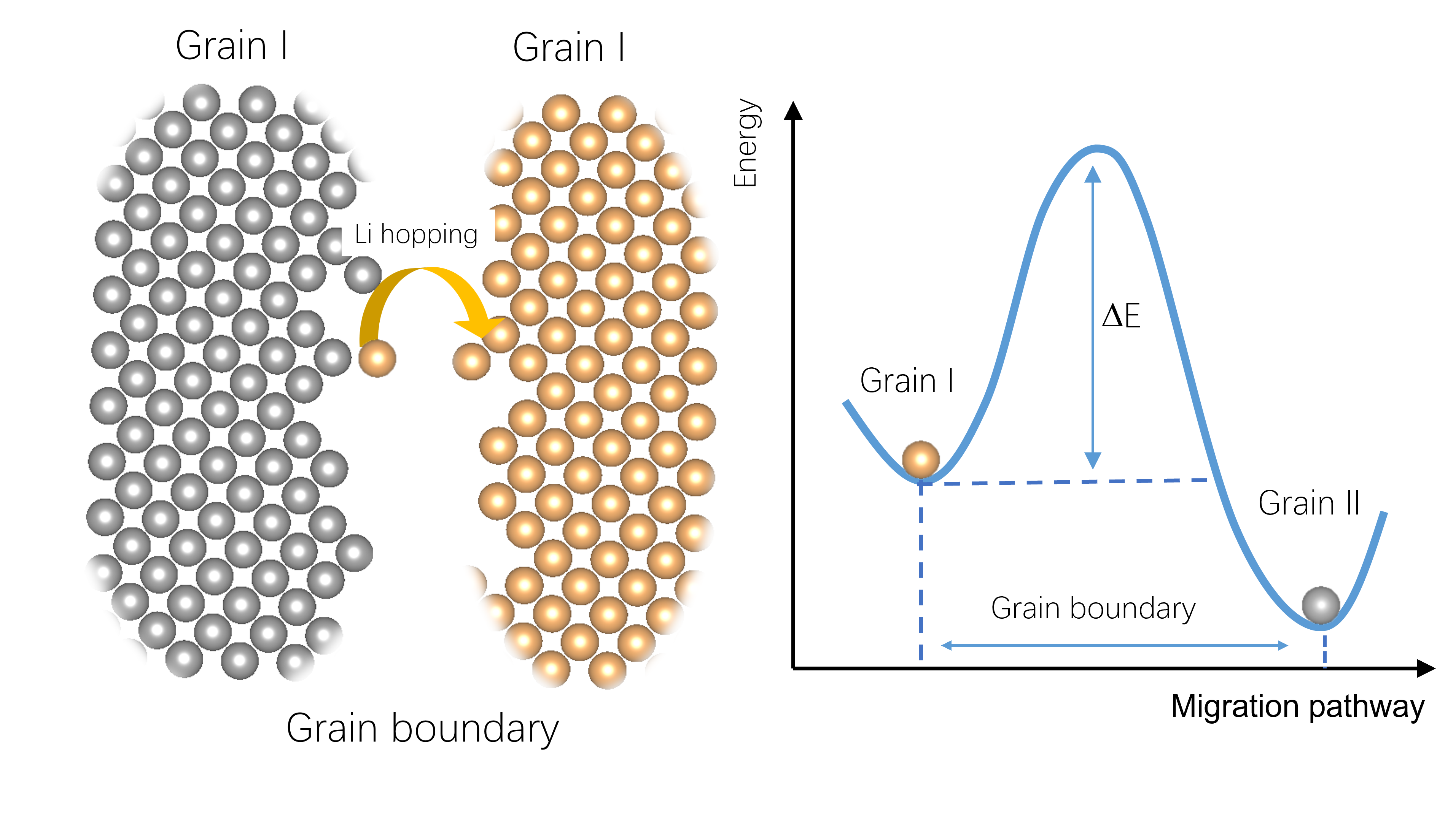
By employing a solid electrolyte, the energy density of a battery can be significantly augmented, primarily through facilitating the utilization of a lithium anode while circumventing the associated risks of lithium dendrite formation. Nevertheless, the solid-solid interface between the electrode and solid electrolyte presents considerable hurdles for efficient lithium diffusion, impeding its practical implementation. Our unwavering commitment lies in comprehending these interfacial challenges to effectively inform the amalgamation of electrodes and electrolytes, thereby fostering the advancement of novel electrode and electrolyte materials.
(3) Understanding battery materials via advanced characterization techniques
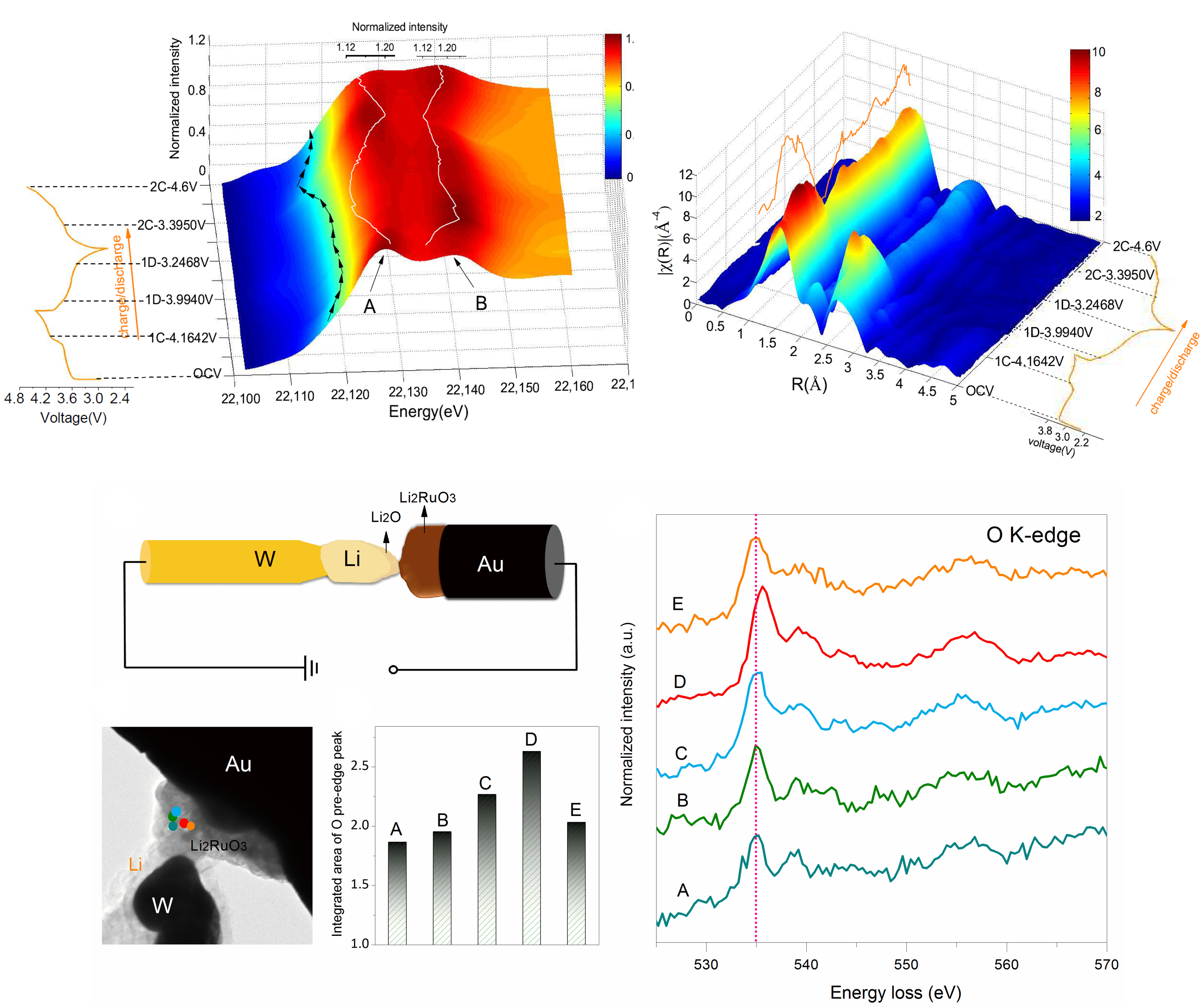
The intricacies of batteries are intertwined with a multitude of occurrences happening within battery materials, necessitating a deeper comprehension attainable solely through advanced characterization techniques. Consequently, there is a pressing need for structurally sensitive methods capable of investigating the evolution of electrode materials at both long-range and short-range scales throughout cycling processes. Furthermore, highly spatially and temporally resolved techniques offer invaluable insights into phase transitions and ionic migration behaviors, which are closely tied to the thermodynamics and kinetics of the materials. Within our laboratory, we not only cultivate our own in-house operando methodologies but also actively seek collaborations with other groups and companies to expand the repertoire of available techniques.